 |
|||
|
Page Title:
Figure 11-6.--Dry-cell battery circuit. |
|
||
| ||||||||||
|
|
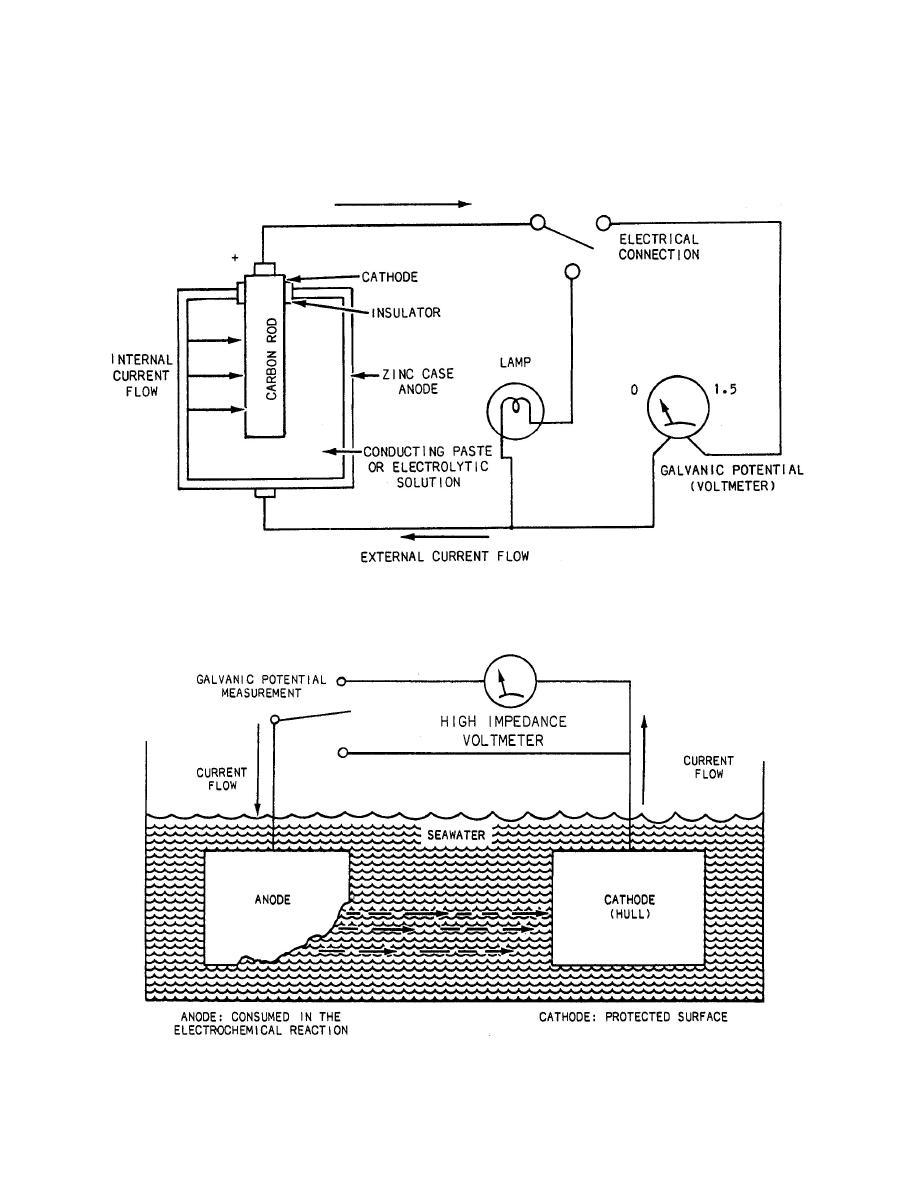 potential of metals. Some of the factors affecting the
than the metal that is less corrosion prone. In
amount of corrosion are stray currents, resistivity,
cathodic protection, the more corrosion-prone metal is
and the temperature of the seawater.
the anode ( zinc) and the less corrosion-prone metal is
Stray-current corrosion is caused by an external
the cathode (steel hull). The rate of corrosion is
current leaving the hull of a vessel and entering the
directly related to the magnitude of the potential
seawater. If the connection between the ship and
difference and is referred to as the open- or half-cell
11-7
|
|
Privacy Statement - Press Release - Copyright Information. - Contact Us |